The 2026 Wall: Why Pharma Feels Stuck (And One Way Out)
Your lab has a molecule problem.
You're after a GLP-1 with less nausea. A kinase inhibitor that spares the heart. An antibiotic bacteria can't outrun.
But the team is stuck: 3-6 months just to map promising changes. $50-100K in consulting fees. Patent uncertainty. The top idea turns out impossible to build. Competitors' failures sit ignored in some old report.
It's not lack of smarts. It's the relay race nobody talks about openly.
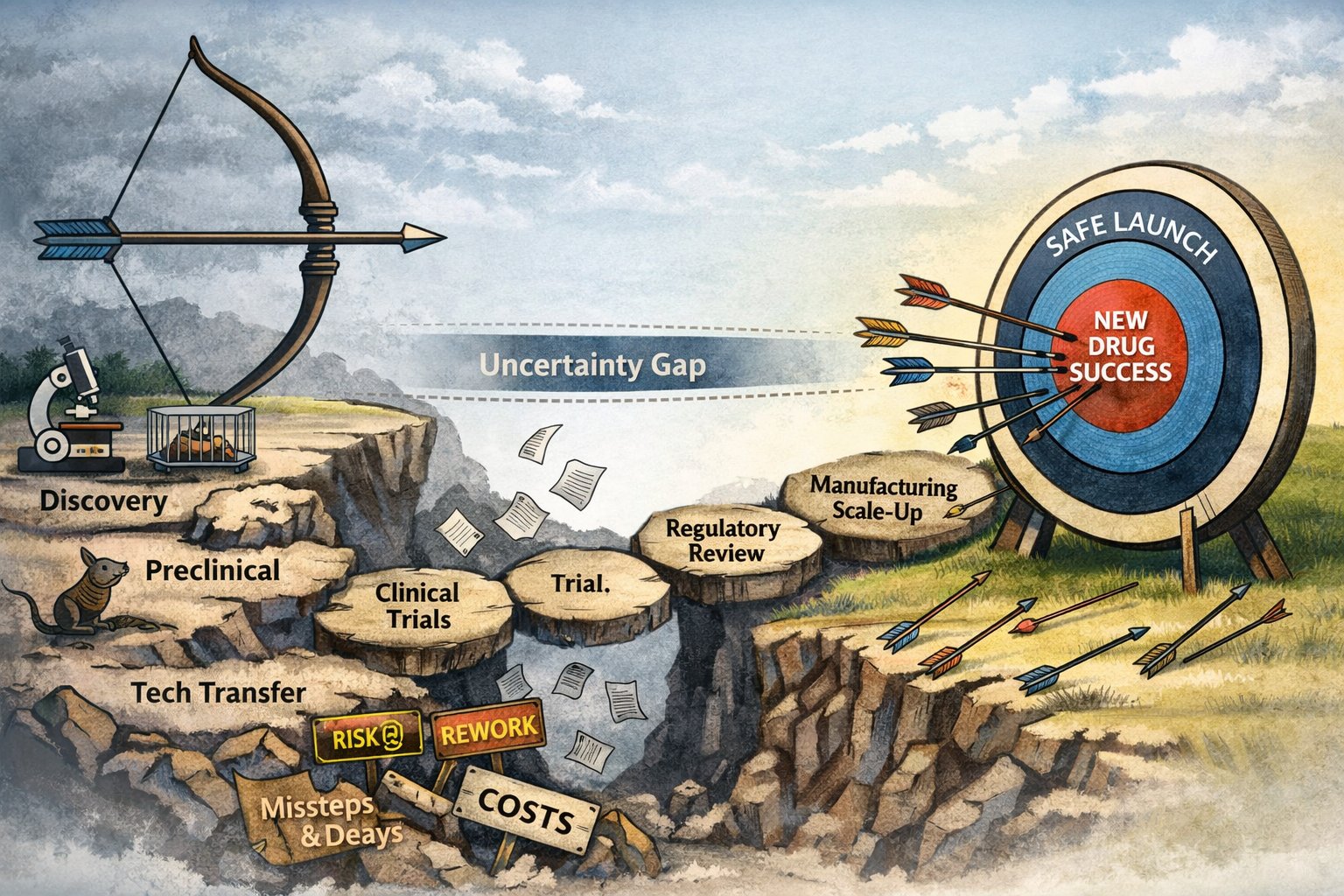
Pharma isn't about "making a drug" in one clean step. It's a long chain of handoffs—discovery to preclinical to trials to regulatory to manufacturing—that stretches 10 to 15 years on average. Costs run hundreds of millions, sometimes billions, depending on what you count.
Each handoff adds its own friction. You finish discovery with a promising hit. Hand it to preclinical—they need toxicity data, formulation tests. That clears, hand it to clinical—now it's dosing studies, patient recruitment, endpoints. Data looks good, hand it to CMC for scale-up: process development, stability, validation.
Every stage builds its own evidence bundle. Regulators don't review "the molecule." They review the full story—how it was made, tested, controlled.
Outsourcing to a CMO or CDMO sounds like a shortcut: "pay money, get product." In practice, it's a careful knowledge transfer. You don't just send a recipe. You transfer the process, the quality system, the impurity profile, the stability plan. The manufacturer runs batches under GMP, documents deviations, qualifies equipment, generates the release data.
Even when smooth, that transfer averages 7 months—sometimes 3 to 15. For complex injectables or biologics, it's often 18 to 30 months or more. Stability studies run alongside, accumulating real-time data. The "product" you get back isn't just material—it's a thick package: batch records, test results, investigations, change controls.
Then regulatory review kicks in: 6 to 10 months standard.
The result? Decisions that feel like they should take weeks stretch into months. Early mistakes—wrong target, overlooked patent, underestimated manufacturing difficulty—don't surface until you've already committed real capital.
2026 turns up the heat. Patent cliffs open wide: semaglutide's protection starts cracking in major volume markets (China, India, others soon after). Generics and biosimilars move fast where they can. The cost of delay jumps—windows narrow for differentiated follow-ons.
Teams feel the squeeze: rush decisions, trust loose summaries, skip full traceability. Money burns quietly on rework, stalled transfers, or late "no-go" calls after too much spent.
CARVE is built for that exact pressure point.
Not a replacement for trials or manufacturing. A fast "should we commit?" layer.
Give us your anchor and goal. 5-7 business days later: structured report, handful of focused options, public evidence, risks as tests, first-pass patent scan (sources logged, limitations clear).
Everything traceable. No black box.
Reserve the slow, expensive machinery for ideas that survive a rigorous first pass.
The wall isn't going away. But decisions can get faster—and smarter.
Pink House Technology Carve
info@pinkhouse.tech
Pre-NDA briefs on request.
